Ozempic & Wegovy Linked to Serious Eye Condition: NAION. Were You Affected?
If you experienced sudden vision loss after using Ozempic, Wegovy, Saxenda, or Victoza, you may have developed NAION - a serious condition that can cause permanent blindness.
Check if you qualify for compensation
Ozempic®, Wegovy®, and Rybelsus® are registered trademarks of Novo Nordisk A/S. This website is not affiliated with, sponsored by, or endorsed by Novo Nordisk.
What to Do If Ozempic or Similar Weight Loss Drugs Caused Your Injury or Illness?
Get Medical Help
If you experience sudden vision changes, eye pain, or vision loss after taking Ozempic, Wegovy, or Rybelsus, seek immediate medical attention. These may be signs of serious eye-related complications.
Secure Medical Records
Ask your medical provider for copies of your medical records, which can serve as valuable documentation when constructing your Ozempic injury claim in the future.
Get FREE Consultation
FREE No Obligation Consultation - Pay Nothing! Your Contact Information and Your Claim are Confidential!
Receive the Compensation
Receive the Compensation You May Deserve! We may be able to help you pursue compensation to pay for your medical bills.
About
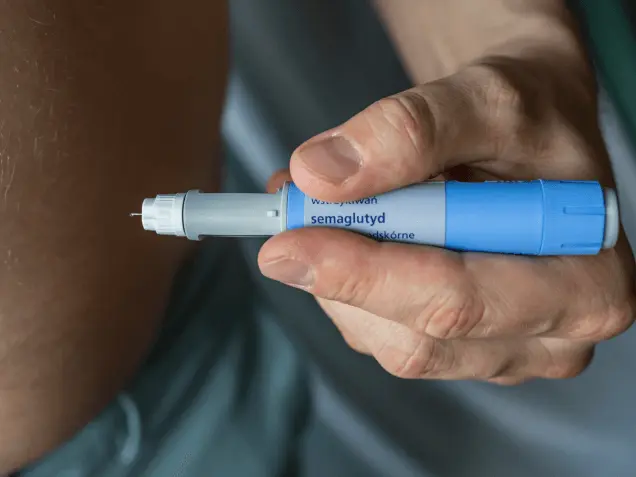
Ozempic, Wegovy, and Rybelsus are diabetes drugs also used for weight loss. Some users have reported serious eye-related side effects, including vision loss.
FDA & Medical Warnings
In June 2022, the FDA flagged serious safety concerns with Ozempic, Wegovy, and similar drugs. Risks include vision loss, NAION, and other severe complications. In May 2023, the National Library of Medicine also reported dangerous side effects. If you've been affected, you may be entitled to compensation.
- FREE, No-Obligation Consultation
- Pay Nothing Unless We Win
- 100% Confidential Case Review
Serious injuries caused by Ozempic use can include:
NAION (Non-arteritic Anterior Ischemic Optic Neuropathy)
NAION is a serious eye condition that can lead to sudden, permanent vision loss. It occurs when blood flow to the optic nerve is disrupted, often without warning.
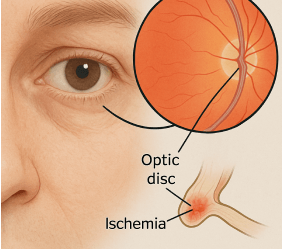
NAION has been reported in patients using Ozempic and similar medications, and may be linked to the drug's impact on blood vessels and circulation.
Symptoms can include blurred vision, partial vision loss, or a dark area in your field of vision. Immediate medical evaluation is critical.
GET HELP NOWEye Stroke
An eye stroke, or retinal artery occlusion, happens when blood flow to the retina is blocked, leading to sudden vision loss.
This condition can be triggered by changes in blood pressure or clotting; both of which may be affected by GLP-1 drugs like Ozempic and Wegovy.
Eye strokes are medical emergencies. If you've experienced sudden vision loss or blurring, seek urgent care and legal guidance.
GET HELP NOWSudden Vision Changes
Sudden vision changes — such as blurriness, double vision, or loss of peripheral sight — may be early signs of serious eye damage.
These symptoms have been reported by users of Ozempic, Wegovy, and Rybelsus, and could indicate underlying conditions like NAION or eye stroke.
Don't ignore changes in your vision. Early intervention is key, and you may be entitled to compensation.
GET HELP NOWSudden Vision Loss
Sudden vision loss is a frightening and potentially permanent condition. It can occur in one or both eyes and may be linked to GLP-1 medications.

Patients taking Ozempic or similar drugs have reported unexpected and rapid loss of sight, sometimes without pain or warning.
If this has happened to you, it's critical to seek medical and legal help immediately.
GET HELP NOW
DON'T LET YOU OR YOUR FAMILY SUFFER IN SILENCE
We may able to help you pursue compensation to pay for your medical bills.
GET HELP NOWIn The News
Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS)
FDA - US Food & Drug Administration
A potentially serious adverse effect of GLP-1 receptor agonists
National Library of Medicine
Woman sues drug-makers of Ozempic and Mounjaro over severe gastrointestinal issues
CNN
Ozempic, Mounjaro manufacturers sued over claims of "stomach paralysis" side effects
CBS News
Makers of Ozempic and Mounjaro sued over 'stomach paralysis' claims
NBC News
Ozempic, Mounjaro makers hit with ‘stomach paralysis’ lawsuit
NY Post
